Reshaping
gynecological
diagnostics.

Who we are
At MiMARK we care for women’s health. We are developing innovative in vitro diagnostic solutions to improve the management of gynecological diseases. We envision the gynecological fluid as the next step in liquid biopsy in gynecology. Our first objective is to improve Endometrial Cancer diagnosis.


Our approach for transforming
gynaecological care
We are developing a solid portfolio of diagnostic solutions based on the use of gynecological fluids, to transform diagnostics for the better.

The future of
Endometrial Cancer Diagnosis


Unmet clinical need
Every year, 35M post-menopausal women worldwide will suffer from Abnormal Uterine Bleeding (AUB). This is the triggering event to start Endometrial Cancer diagnosis. Starting the diagnosis of endometrial cancer is mandatory since 5% of these women will suffer from it. The current diagnosis is based on the pathological examination (observation of cells) of an endometrial biopsy taken by aspiration, a minimally invasive procedure. However, the analysis of this sample fails to diagnose 31% of patients due to the scarce cellularity of the sample. In those cases, invasive diagnosis using hysteroscopy needs to be performed.
ENDOMETRIAL CANCER
1st
gynecological cancer in
high-income countries
Half a million
new cases every year
+46%
incidence by 2040

In Vitro Diagnostics (IVD) immunoassay to aid in Endometrial Cancer Diagnosis for post-menopausal women with AUB referred to Endometrial Biopsy.
Our solution: WomEC
WomEC is an IVD immunoassay to aid in diagnosing Endometrial Cancer based on quantifying a panel of protein biomarkers in the uterine fluid. The sample that WomEC utilizes is the minimally invasive endometrial biopsy by aspiration. But in this case, no cellular material is required. By using uterine fluid rather than cellular material for EC diagnosis, WomEC can provide a highly accurate diagnosis. We are targeting a 97% sensitivity and a 97% negative predictive value.
WomEC is being developed as an immunoassay test to facilitate its implementation in clinical laboratories.
How will it impact the healthcare system
To PAYERS
More efficient patient management: reduced unnecessary procedures
Annual cost reduction in the US (reduction of hysteroscopies). The average saving per patient is 1.196$.

| OUTCOMES | IMPACT |
|---|---|
| Two EU patents have been already accepted in several regions. | Well protected asset |
| Eight retrospective studies involving nearly 1000 patients using mass spectrometry (LC-PRM) technology and ELISA assay | Protein accuracy in clinical setting. |
| International publications:
– Development of a sequential workflow based on LC-PRM for the verification of endometrial cancer protein biomarkers in uterine aspirate samples. Oncotarget 2016. |
Scientific validation |
| Proprietary Key Reagents developed | Prototype feasibility |
| Validation of the unmet clinical need and WomEC value with more than 250 gynecologists and pathologists (primary qualitative research and primary quantitative research).
The clear majority (92%) of MDs interviewed can see themselves using our IVD in the future and see value in our product. |
Clinical relevance |
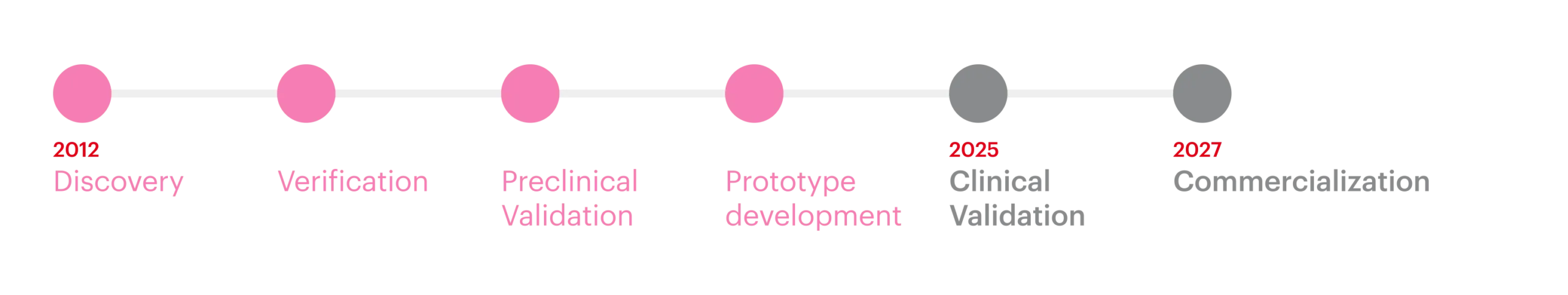
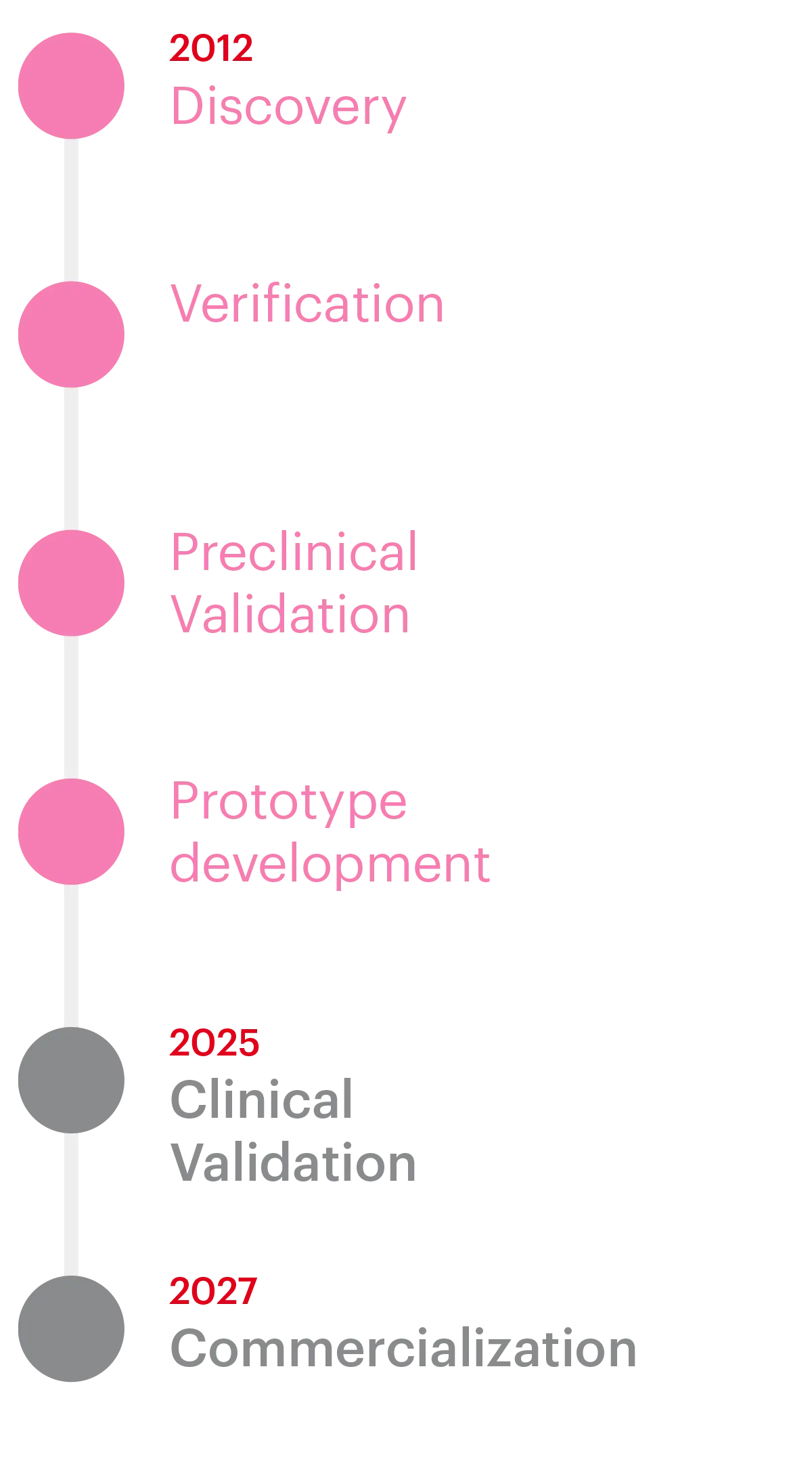
| Currently finishing the prototype development stage with proprietary antibodies |
Prototype development |
We seek partners to foster WomEC development,
validation and commercialization
Key Opinion Leaders are supporting WomEC

“WomEC will revolutionize uterine cancer diagnosis in the world”
Javier F. Magrina,
Director Gynecologic Oncology

“From a clinical perspective, the project is expected to benefit the European community”
Frederic Amant,
Head of the gynecology oncology department,
ex-chairman of the ENITEC consortium
WomEC – an EIC funded innovation
We are proud to announce that WomEC was awarded €2.5 million by the European Commission through the prestigious EIC Accelerator program (March 2023 cohort). This significant funding supports the completion of WomEC’s development and the clinical validation necessary to bring our innovative Endometrial Cancer diagnostic solution to market.
The project, which officially began in October 2023, is scheduled to run until the end of 2025. During this time, our team will focus on completing the development WomEC immunoassay and ensuring it meets the highest standards for clinical effectiveness and patient impact.
For more information please click here.
MiMARK recognition:
Awards and Honors
NOTABLE AWARDS

Extraordinary price in Health Technologies by the Fundación para la Excelencia y la Calidad de la Oncología (ECO), 2022

STEM Women Congress Award Spain, 2022

Women Startup Awards in the Early-stage category from WomenInTechSpain, 2022

Winners of e-llas Innovation Hub, GSK, 2024

MiMARK as one of Spain’s most innovative companies
PITCH COMPETITION SUCCESS

Agnes Guerraz Prize by the WorkInHealth Foundation from EIT Health, 2023

Selected as one of the 100 start-ups to pitch on stage during the 2023 edition

Winners of Pitch competition of the Gender Gap in Investment Event, 2024
Investors:
 |
 |
 |
 |
 |
 |
 |
Public Funding Sources:
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
Partners:
 |
 |
 |
 |
 |




















